
Choosing the right AAV serotype can define a gene delivery project’s success. Get it wrong, and you face low efficiency, off-target effects, or wasted effort. For teams developing gene therapies or otherwise leveraging the AAV technology, this decision shapes your workflow from day one. AAVs offer versatility, but their diversity—spanning natural and engineered variants—demands careful planning to match serotypes to your target tissue and application. A misstep here isn’t just a hiccup; it can significantly derail your timelines and budgets. Start smart, and even small teams can deliver big results owing to the unique characteristics of this powerful gene delivery tool. This guide is an evolving resource that keeps you up to date with the essentials: a concise history, serotypes tailored to key tissues, and an preview of capsid engineering and optimization. Whether you’re new to AAVs or refining your approach, understanding these options sets you up for success.
A Brief History of AAV Serotypes
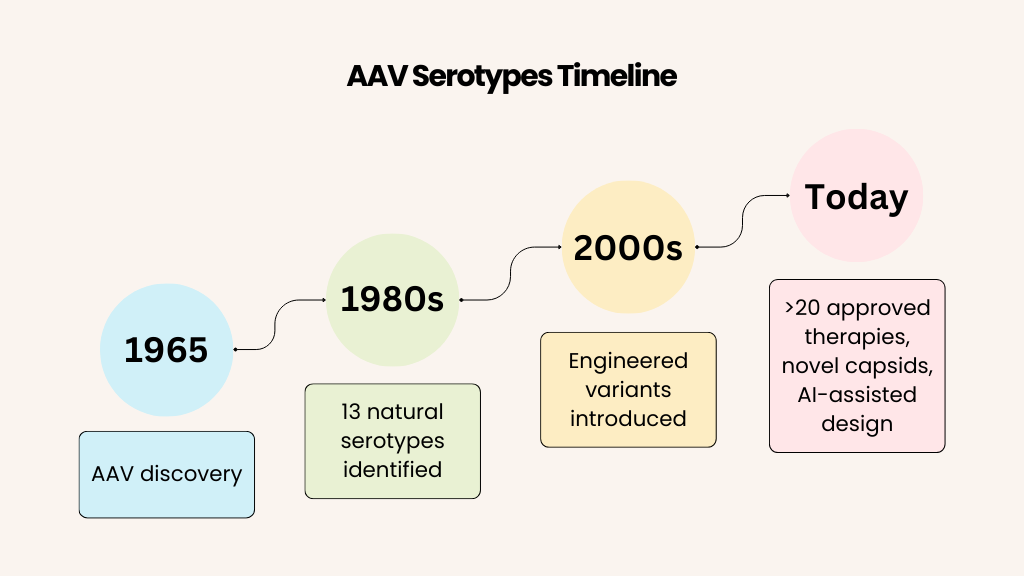
AAVs first appeared in scientific literature in 1965 when they were identified as contaminants in adenovirus preparations from human and monkey samples. These small, non-enveloped viruses, part of the Parvoviridae family, quickly intrigued researchers due to their single-stranded DNA genome and icosahedral capsid. Over the following decades, 13 natural serotypes (AAV1-13) were identified, each distinguished by variations in capsid proteins that influenced their tissue tropism. AAV2, the first extensively characterized serotype, became the benchmark due to its broad transduction capabilities.
By the 1980s, AAVs emerged as promising gene delivery vectors, valued for their safety, low immunogenicity, and ability to provide long-term gene expression in non-dividing cells. However, the limitations of natural serotypes—such as variable specificity and immune responses—drove innovation. Beginning in the 2000s, researchers engineered variants like AAV-DJ and AAV-PHP.B, modifying capsids to enhance targeting efficiency and expand therapeutic possibilities. This evolution continues today, with natural and engineered AAVs offering an ever-expanding toolkit for gene therapy.
For more background, see our Introduction to AAVs.
Serotypes by Tissue Targets
Selecting the right serotype means matching it to your target tissue. Below are key categories and the best-suited serotypes for each.
Musculoskeletal (e.g., Muscular Dystrophies)
Muscular dystrophies, such as Duchenne muscular dystrophy (DMD) or Limb-girdle Muscular Dysrophy (LGMD), require highly efficient muscle transduction. AAV1 and AAV6 bind sialic acid on skeletal and cardiac myocytes, ensuring robust gene expression, while AAV6’s heparin affinity provides an additional advantage. AAV8 and AAV9 leverage galactose receptors for broader muscle penetration, making them ideal for systemic applications. AAVrh74, derived from rhesus monkeys, offers enhanced specificity through capsid modifications. More recently, engineered variants like MyoAAV and AAVMYO incorporate RGD peptides into VP3 surface loops, binding integrins on muscle cells for superior targeting. These serotypes provide critical options for muscle-focused therapies, particularly for delivering large genes like dystrophin, which often require split vectors.
Central Nervous System (e.g., Neurological Diseases)
For neurological diseases like ALS or Parkinson’s, precision in CNS targeting is paramount. AAV9 crosses the blood-brain barrier (BBB) using N-linked galactose, efficiently transducing neurons and glia, making it a go-to for brain-targeted research. AAV5 preferentially transduces astroglia through sialic acid interactions, making it an excellent choice for glial-focused strategies. AAV2-Retro, engineered with retrograde transport peptides in VP1, enables efficient tracing of neural circuits by moving from axons to cell bodies. Additionally, AAV-PHP.B and its improved variant PHP.eB incorporate surface loop modifications at the VP3 588 site, dramatically enhancing BBB penetration for systemic neurological applications. These serotypes provide a range of options for CNS-directed gene therapy.
Hematopoietic Cells (e.g., Blood Disorders and Gene Editing)
For hematopoietic applications such as leukemia treatments and CAR-T cell engineering, serotype selection is crucial for effective gene transfer in vivo and ex vivo. AAV6 is well-suited for hematopoietic stem and progenitor cells (HSPCs), due to its affinity for sialic acid receptors to achieve high transduction rates. AAV3B binds heparan sulfate glycosaminoglycans (HSG), making it a strong candidate for targeting leukocytes. Chimeric serotypes like AAV-DJ, which blends elements of AAV2, AAV8, and AAV9, offer enhanced transduction across multiple cell types, making them valuable for ex vivo applications. AAV10, though less studied, has shown promise in stem cell applications. Selecting the right serotype early has proved essential for optimizing gene delivery efficiency in hematopoietic projects.
Liver (e.g., Metabolic Disorders)
For liver-directed therapies such as hemophilia treatments or metabolic disease interventions, hepatic tropism and low toxicity are key. AAV8 has emerged as a top performer, efficiently transducing hepatocytes, likely through interactions with laminin receptors (LamR). AAV7 exhibits similar behavior but remains less characterized. Engineered hybrids such as AAV-DJ/8 improve upon these natural serotypes, offering enhanced liver specificity and higher gene expression levels. These serotypes enable effective systemic gene therapies that rely on stable hepatic gene expression for months or years.
Ocular (e.g., Retinal Disorders)
Gene therapies for retinal diseases require vectors that efficiently transduce different ocular compartments. AAV2 remains a gold standard for retinal pigment epithelium (RPE) targeting, with widespread clinical use in inherited retinal disorders. AAV8 and AAV9 demonstrate strong retinal penetration and are often used for photoreceptor and inner retinal targeting. Variants like AAV7m8, developed through directed evolution, improve retinal transduction efficiency, particularly for subretinal delivery. These serotypes form the backbone of ocular gene therapy research and development.
AAV Capsid Engineering: Expanding the Toolbox
While natural AAV serotypes provide a strong foundation, engineering has unlocked new possibilities for improved targeting, immune evasion, and efficiency. Directed evolution—where capsid libraries are screened in vitro or in vivo—has yielded highly optimized variants such as AAV-PHP.eB for CNS delivery and MyoAAV for muscle targeting. Specific mutations, such as those modifying receptor-binding domains, can enhance tropism or immune evasion. Additionally, inserting peptides into surface loops allows for receptor-specific targeting, broadening the utility of AAVs for precision gene therapy. A more in-depth exploration of capsid engineering will be covered in a future post.


